Some chelated minerals tend not to break apart during digestion, forcing the metal mineral ion to go where its chelating "partner" goes
Reference: https://www.wattagnet.com/articles/410-chelates-clarity-in-the-confusion
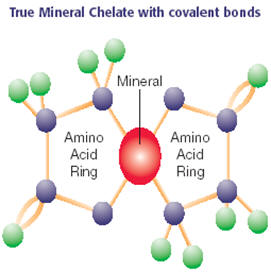
What is a "chelate" in simple terms: A chelate means to create a ring-like complex where a metal ion grabs and bonds to another ion or organic molecule. Chelated mineral supplements, usually with the mineral bonded to an amino acid, are touted for their improved absorption since the mineral tends not to break off from its "partner" until it is absorbed across the intestinal wall.

 hydroxide
(OH-), Ammonia (NH3), water, carbon monoxide, chloride, fluoride,
iodide, and bromide ions.
hydroxide
(OH-), Ammonia (NH3), water, carbon monoxide, chloride, fluoride,
iodide, and bromide ions.Complexes resulting from coordinate bonds with a chelating ligand are usually more thermodynamically stable than complexes with NON-chelating ligands
ORGANIC ACIDS (including AMINO ACIDS), SMALL PEPTIDES (and some other organic molecules, such as polysaccharides) can form CHELATES with their heterocyclic rings (noting that some chelations have more stability than others - see below) - amino acids form chelates by forming a ring structure via multiple coordinate covalent bonds Eg. Attaching the two ends of an amino acid (an oxygen atom of its carboxylic group end and the nitrogen atom of the amino group end) to the metal mineral ion. Small peptides from protein digestion also have at least two functional groups (amino and hydroxyl) to form a ring with a metal mineral ion.
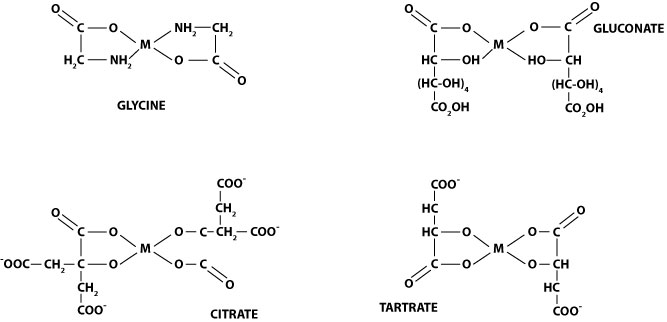
Example chelated minerals - in the diagram below, "M" represents a metal mineral. Gluconate, citrate and tartrate are organic acids, glycine is an amino acid (a specific organic acid - the building block of proteins).
Chelated minerals found in nature. Some examples are magnesium-binding chlorophyll (a plant can synthesize chlorophyll using the amino acid glycine), vitamin B12 (contains cobalt), iron-binding haemoglobin and cytochrome (contains iron); upon entering a plant from the soil, a metal mineral will form chelates with organic acids, such as citrate, malonic and gluconic acids and some amino acids.
Chelates can also be produced under controlled conditions - by reacting inorganic mineral salts with, for example, an enzymatically prepared mixture of amino acids and small peptides; the transition metals (Eg zinc, copper, iron, manganese) can best form stable coordinate covalent bonds with amino acids and peptides, but non-transition metals (Eg. magnesium, calcium} can also form complexes with suitable ligands; Examples of amino acid chelates include: magnesium glycinate, zinc methionate, zinc glycinate; The advantage of metal-amino acid chelated supplements are that they can be recognized and absorbed as food.
Factors for producing a chelated metal/mineral are:
So-called "true" chelates contain coordinate bonds stable enough to endure the journey through GI tract (which includes the HCl in the stomach) without breaking down - which would otherwise increase the chance of negative reactions with other substances in the GI tract before the chelated molecule is transported from the intestines. The "Stability Constant" between the specific metal and specific ligand in the complex determines the likelihood of the mineral staying with its chelating ligand:
More technical details for those who seek the nitty-gritty :)
Peptide complexes make up proteins, and are the mineral forms naturally found in food
WILL LIKELY STAY INTACT - HCl unfolds amino acids in proteins and activates pepsin enzymes, which break them down into shorter amino acid chains, called peptides. Peptides enter the intestines intact, where pancreatic enymes will further break them down into amino acids, which can be transported intact through the intestinal wall and into the bloodstream.
A non-soluble amino acid chelate with its strong bonds and insolubility are considered more bioavailable than inorganic or organic mineral salts, since they do not rely on solubilty (whereby the mineral ion and its partner ligand separate into ions), and the mineral is transported through dedicated amino acid transport sytems.
MOST WILL BREAK DOWN IN GI tract - most organic acids are weak acids, which can be dissolved by HCl
Includes: picolinic acid, citratic acid, malic acid, ascorbic acid, lactic acid, tartric acid, succinic acid, orotic acid;
Example supplemental salt forms: magnesium malate, zinc citrate, calcium citrate, magnesium orotate, zinc picolinate, zinc gluconate
IMPORTANT EXCEPTIONS:
Phytic acid - certain metallic mineral ions (calcium, magnesium, iron, zinc, selenium, chromium, and manganese) bonded to phytic acids can not easily be separated and usually end up being expelled as waste. Phytate (its minerally chelated form) is found particularly in the husks of grains, nuts, seeds and legumes, which must be properly prepared by soaking, souring or sprouting before consumption to release their chelated mineral
Oxalic acid - can irreversibly bind certain minerals in foods such as rhubard and spinach
WILL LIKELY BREAK DOWN IN GI tract - Eg. polysaccharides (complexes of a soluble salt with simple sugars) are larger molecules, which lack stability
Neutral amino acids (Ala, Val, Leu, Met, Phe, Tyr, Ile);Basic amino acids (Lys, Arg) and Cys; Imino acids and Glycine; Acidic amino acids (Asp, Glu) ; Beta amino acids ( beta Ala)
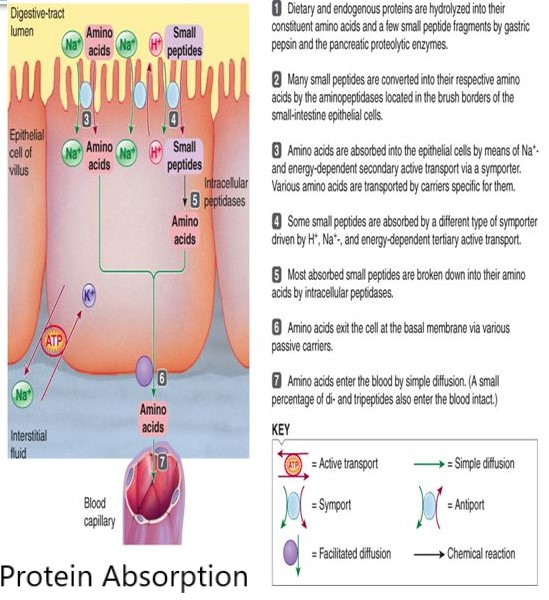
To be absorbed, a chelated molecule must first be moved from the intestinal lumen and enter an intestinal mucosal cell, which is acieved via either:
A summary of Professor D.S. Parsons, the chair at a 1976 international conference on intestinal permeation is given by Dr. H. Dewayne Ashmead, current president of Albion Laboratories, in his book, Amino Acid Chelation in Human and Animal Nutrition
Any of several carrier molecules (including water) must be attached to an amino acid chelate to transport it intact across the luminal mucosal membrane into the mucosal cell. The carrier can be water if the amino acid chelate is dissolved in that water. Study data on amino acid chelate absorption generally demonstrates a regulatory mechanism to control the chelate's rate and amount of absorption into the mucosal cell in facilitated diffusion (transport via a protein channel without using energy, as a substance moves from a higher to lower concentration i.e. down a concentration gradient) but not in simple diffusion. i.e in facilitated diffusion the rate of absorption decreases as the concentration in the mucosal cell increases; until the concentration tends towards equalization on either side of the membrane.
The more soluble amino acid ligands have higher absorption rates via facilitated diffusion - A 1991 Albion Laboratory study using rats comparing uptake of amino acid chelated minerals zinc methionine to zinc glycinate in the small intestine concluded that absorption into blood was largely by facilitated diffusion of the intact chelates. Just as significant, the more soluble amino acid chelate (which is zinc glycinate, because the ligand glycine is more soluble than methionine), was absorbed at a greater rate into the blood, and differences were also reflected in organ tissues. It is emphasized that this study only shows that absorption rate via facilitated diffusion is increased with a chelate ligand with higher solubility, and does not address absorption differences by any active transport (which is likely to occur if lumen pH is low enough to dissociate the mineral ion from its chelated amino acid).
AMINO ACID CHELATES |
||
| Magnesium bonded to an amino acid (C,H,O,N; E.g. glycine is C4H8N3O2); STRONG chelators; rely on amino aid pathways rather than their solubility. Theoretically these may have advantageous absorption over other supplements due to (1) Not dissociating in the stomach, preventing the still bonded mg ions from otherwise forming less separable bonds with phytic acid and/or (2) being absorbed intact via amino acid pathways in the gut lining | ||
| Magnesium AMINO ACID CHELATE | Elem. Mg % | Comments |
| Magnesium glycinate | 11-14% or 18% |
3.45 aka magnesium bisglycinate; glycine is a small, calming amino acid |
| Magnesium lysinate | ||
| Magnesium taurinate | 8.8% | Both magnesium and the amino acid taurine: benefit cardiovascular health (improve cardiac function, reduce blood pressure, stabiize heart muscle contraction, anti-thrombotic), improve insulin sensitivity, and calm neuromuscular excitability. |
| Magnesium glutamate | ***AVOID: Glutamate is an excitory neurotransmitter; Neurotoxic (see below) | |
| Magnesium aspartate | 7% | ***AVOID: Aspartate is an excitory neurotransmitter; Neurotoxic (see below) |
| Magnesium L-Threonate | 8.1% | Passes BBB; improve memory and brain function.
One preliminary study in animals found that it significantly enhanced both short-term and long-term memory, boosting scores by 15% for short-term memory and 54% for long-term memory compared to magnesium citrate. Slutsky I, Abumaria N, Wu LJ, et al. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010 Jan 28;65(2):165-77 Superior ability to penetrate the mitochondrial membrane, |
| Magnesium Arginate | ||
### a href="http://en.wikipedia.org/wiki/Magnesium_citrate" title="Magnesium citrate"> Magnesium citrate has been reported as more bioavailable than oxide or amino-acid chelates: glycinate, aspartate (neurotoxic), taurinate, lysinate, arginate, or organic acid chelates: orotate or succinate forms.
Walker AF, Marakis G, Christie S, Byng M (2003). "Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study". Magnes Res 16(3): 183-91. PMID14596323
***Both magnesium aspartate and magnesium glutamate break down into neurotransmitters that, when not bound with other amino acids, are neurotoxic.
https://www.wattagnet.com/articles/410-chelates-clarity-in-the-confusion