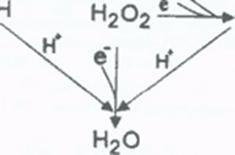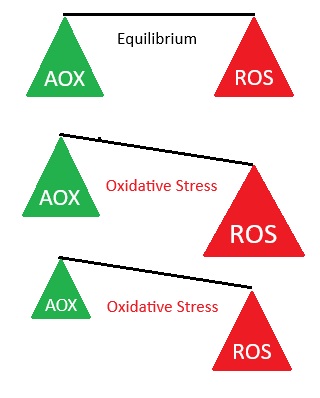
Antioxidants are needed to balance the oxidants produced by and present in the body, preventing cellular damage that initiates many diseases. Normal biological activities of aerobic cells and organisms continuously generate free radicals (oxidants). In humans, the most important are reactive oxygen species (ROS), such as singlet oxygen, hydrogen peroxide , superoxides, and hydroxyl ions, which can inflict cellular damage when they are generated to the extent that they overwhelm the cells' ROS "damage control" antioxidant systems. Oxidant / antioxidant balance i.e. maintaining oxygen homeostasis (stability), is the major factor in maintaining good cellular function and preventing oxidative stress, which plays a significant role in the progression of many diseases, including diabetes, Alzheimer disease, and atherosclerosis / heart disease.

To prevent damage to cells in the body from excess ROS, we need to:
ROS:: have a paradoxical presence - although necessary for health, if uncontrolled they can also cause many of today's familiar diseases:
Life's Oxygen Paradox - Meet Dr.ROS Jeckyll and Mr.ROS Hyde
Antioxidants sacrifice themselves to preserve your body parts - basically, antioxidants are molecules that ROS, such as free radicals, find "more attractive"than cellular components. Antioxidants readily donate their electrons to prevent free radicals from stealing electrons out of membrane fatty acids, mitochondria, DNA, and elsewhere. After donating an electron, an antioxidant becomes a free radical by definition. Antioxidants in this state are not harmful because they have the ability to accommodate the change in electrons without becoming reactive themselves.
Antioxidants each work in their own unique manner and in their particular area of expertise, but they also complement each other - in a wonderful synergy that effectively protects cells from free radicals.
"When vitamin E disarms a free radical, it becomes a weak free radical itself. But unlike other free radicals, the vitamin E radical can be recycled, or turned back into an antioxidant, by vitamin C or coenzyme Q10. These network antioxidants will donate electrons to vitamin E, bringing it back to its antioxidant state."
- Dr. Packer, author of "The Antioxidant Miracle"
Healthy cells and aerobicorganisms have more protectiveantioxidant enzymes (E.g. SOD, CAT and Glutathione peroxidase) than anaerobic organisms - which are thus more vulnerable to oxidative damage.
There are two types of antioxidant protective mechanisms against oxidative stress - Enzymatic and Non-Enzymatic
All antioxidants need optimal amounts of certain dietary, MINERALS to function properly
Superoxide Dismutase (SOD). This water-soluble enzyme needsmanganese, zinc, copper (marginal to deficient in US diet), depending on location (Zinc-Copper-SOD in the cytosol, Manganese-SOD in the mitochondria.
|
O2•- + O2•- |
→ |
O2 + H2O2 |
|
Mn / Zn or Cu-SOD |
Catalase (CAT). Found in membranes of eukaryotic cells (thus not in bacteria); Requires iron to function
2H2O2 → O2 + 2H2O
Glutathione peroxidase (GPx) Contains and requires selenium to function; actually a family of 8 isoforms: Gpx1 (in cytoplasm of most all mammalian cells), GPx2 (intestines) GPx3 thru GPx8, each found in specific areas of the body). Has two main functions in reactions requiring glutathione (GSH):
|
2GSH + ROOH |
→ |
GS-SG + ROH + H2O |
|
|
GPx |
|
|
2GSH + H2O2 |
→ |
GS-SG + 2 H2O |
|
|
GPx |
|
Protect aqueous areas (most of the cell's cytoplasm) from oxidation
Vitamin C (Ascorbic Acid / Ascorbate)
Glutathione (GSH) (requires selenium) - PRIMARY antioxidant and detoxifier in cell's cytoplasm.
For more details on this major antioxidant and how to increase your body's intracellular levels:
Glutathione - "King of the Antioxidants"
Powerful radical scavengers protect lipid membranes from oxidation
Generally, these are phenols that donate a hydrogen atom to a peroxyl radical, converting it to a lipid hydroperoxide.
Vitamin A / Carotenoids (E.g β-carotene (with fat can be converted to Vitamin A), lutein, zeaxanthin and lycopene);
Vitamin A - "The Grass Vitamin"
Vitamin E (Tocopherols)
CoEnzyme Q-10 (a phenol)
Vitamin D
Vitamin D -"The Sunshine Vitamin"
Vitamin K
Vitamin K -"For Klotting and Kalcium"
MELATONIN
MELATONIN - "Darkness Hormone"
Uric Acid (most important plasma antioxidant in humans)
Flavonoids
Lipoic Acid
Bilirubin / Histidine - singlet oxygen quenchers
Refers to the minimum distance away a free radical scavenger must be from a radical to be able to neutralize the radical.
E.g. OH•
travels no more than several Angstroms before it interacts with another molecule
and a free radical scavenger must be within this
"cage" to neutralize the radical. Thus, for example,
if an antioxidant is confined to the lipid-rich
membrane of a cell because of its specific solubility, it will be ineffective in
reducing OH•
damage to DNA in the nucleus.