
Antioxidants -"Oxidant damage control"
The oxygen we breathe into our bodies oxygenates and energizes our cells, providing acute life-support for our tissues and organs. The oxygen molecule is utilized by being chemically changed into different "reactive" molecules called reactive oxygen species (ROS). ROS have become a subject of great interest due to their implication in numerous diseases of our time, including rheumatoid arthritis, Alzheimer's and Parkinson's disease, high blood pressure, atherosclerosis, liver cell injury, cancer, Type 2 diabetes and more. However, ROS should not be thought of as "bad", since they are formed as necessary intermediates in a variety of normal biochemical reactions in the body.
Where do body's ROS come from?
ROS are absolutely necessary for day-to-day functions in the body for life and health ( represented by Dr ROS Jeckyll). E.g.ROS are part of the process of digesting food, necessary for generating of ATP (cellular energy); Immune system macrophages and neutrophils use ROS to destroy foreign organisms
However, uncontrolled ROS can also be toxic to cells and cause harm to the body (represented by Mr. ROS Hyde). Proteins, membrane lipids, carbohydrates and nucleic acids (DNA, RNA) are subject to cellular damage by ROS, and are implicated in many chronic diseases.
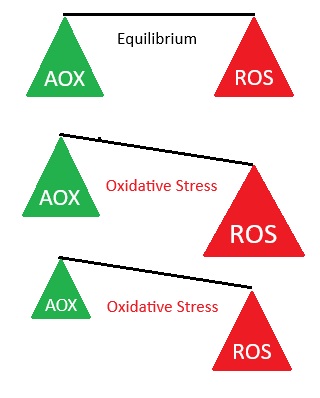
ROS are removed by protective enzymes and antioxidants
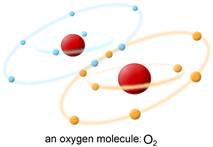
The oxygen molecule found in the air we breathe is written chemically as 3O2. The "2" in 3O2 refers to the two chemically bonded oxygen atoms which make up an oxygen molecule. The "3" refers to it being in the triplet or ground state, meaning its outer (valence) electrons are spinning in parallel, i.e. in the same direction, in separate orbitals.
The 3O2 molecule found in the air is not very reactive because it is a di-radical. So named because it has two electrons in different orbitals without a "mate", making it "hungry" for two electrons (e-). In fact, the oxygen molecule has TWO unpaired electrons WITH PARALLEL SPINS. This makes 3O2 very unlikely to participate in reactions with our body's organic molecules, since organic (i.e. carbon-based) molecules that serve as substrates for oxidation do not contain unpaired electrons with their bonds in stable form, having two electrons with antiparallel spins. (Pauli's exclusion principle only allows 3O2 to be an oxidant in the rare case that the reductant (the molecule supplying the electrons) also has two unpaired electrons, with parallel spins opposite to that of oxygen).
The usefulness of the 3O2 oxygen molecule, with its two unpaired electrons, is in its ability to be "activated" to produce more reactive molecules than itself.
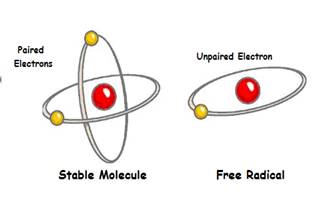
ROS are small ions or molecules with one or more unpaired electrons in their outer orbital(s) making it highly reactive and likely to take part in chemical reactions with your body's proteins, lipids, carbohydrates and DNA. Electrons within atoms and molecules occupy orbital spaces, in which each orbit holds a maximum of two electrons. Electrons prefer to exist in pairs, and if an electron does not have a "mate" with which to share its orbital space, it will endeavor to "steal" one from another atom (technically, it will oxidize another atom). An ROS can have more than one unpaired electron.
ROS are formed by the loss or addition of an electron from a stable molecule in a REDOX (REDuction / OXidation) reaction. This chemical term refers to a two-way reaction in which
E.g. Oxygen can be activated by receiving single electrons by chemical transfer, as in metabolism.
(For the sake of completeness, another way an ROS is formed is when a normal molecule is split into two fragments - each retaining one of a previous pair of electrons. This is not common in the body, since it needs high energy input E.g. UV and ionizing radiation can produce the very reactive singlet oxygen (1O2* ) when one of oxygen's electrons does a spin flip and jumps to a higher orbital following absorption of energy).
A chain reaction forms many cell-damaging ROS. A newly formed ROS quickly "steals" an electron(s) from a nearby molecule, which satisfies its need for a pair (or pairs) of them in its outer orbital(s), but which then turns its "victim" into an ROS. A chain reaction is thus proliferated, resulting in the damage and disruption of a living cell.
ROS can have positive, negative or neutral charge. ROS identity only concerns the arrangement of its electrons in its outer orbital(s). Charge is determined by the difference between the number of protons and electrons. E.g. A neutral molecule could have gained or lost an electron. A non-neutral molecule could become neutral with the addition or loss of an electron.
ROS are categorized as either radicals (sometimes called free radicals) or non-radicals - both of which are reactive. The difference - a radical is a covalently bonded group of atoms that take part in a reaction as a single unit, a non-radical does not. E.g. Superoxide and hydroxyl are radical ROS, hydrogen peroxide, peroxynitrite and singlet oxygen are non-radical ROS.
The most important radicals in the body concerning our health are derivatives of oxygen. However, radicals can also be non-oxygen-centered:
Free radicals are frequently denoted by a dot placed immediately to the upper right of the atomic symbol or molecular formula. The radicals of most concern to the body are the hydroxyl radical (OH•), alkoxy radical (LO•) and superoxide radical (O2•-).
| Biologically significant ROS (derived from oxygen) | |||
|---|---|---|---|
| ROS | Radical | Symbol | |
| Hydroxyl radical | ✓ | OH• | |
| Alkoxy radical | ✓ | LO• | |
| Superoxide radical (anion) | ✓ |  |
O2- |
| Hydroxyl anion | Not | OH- | |
| Peroxyl radical | ✓ | LOO• | |
| Hydrogen peroxide | Not | H2O2 | |
| Singlet oxygen | Not | 1O2 | |
| Ozone | Not | O3 | |
| Nitric oxide | ✓ | NO• | |
| Peroxynitrite (anion) | Not | ONOO- | |
| Hypochlorous acid | Not | HOCl | |
| Hydroperoxide | ✓ | LOOH | |
| Chlorine radical | ✓ | Cl• | |
In biological systems, REDOX reactions using the oxidant ability of ROS are essential to body's functions for maintaining life. ROS are produced continuously in cells either as by-products of metabolism or deliberately as in immune system phagocytosis. They are also by-products of circulation, respiration, digestion and assimilation.

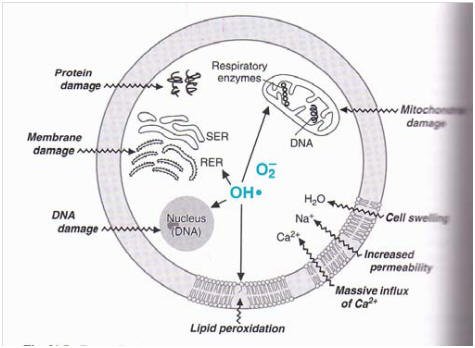
ROS mediate cellular injury. Superoxide O2• -and hydroxyl OH• radicals initiate peroxidation in the cellular, mitochondrial, nuclear, and endoplasmic reticulum membranes. E.g. This increases the cellular permeability for Ca2+ ions whose increased cellular concentrations damage the mitochondria.
Uncontrolled ROS play a role in disease and degenerative conditions

Most radicals can pull an electron from (i.e. oxidize) most biochemical compounds. In trying to gain stability, by "stealing" (or donating) an electron, ROS radicals quickly react with most nearby molecules, including proteins, lipids, carbohydrates and DNA. When the "attacked" molecule loses (or gains) an electron, it becomes a radical itself, beginning a chain reaction which can result in the disruption of a living cell. The amount of damage incurred is dependent upon both the number of radical hits, and the amount of protection by the antioxidant "damage control" system
While oxygen and its ROS offspring are an essential part of many, normal body processes, aerobic cells may experience oxidative stress damage if their antioxidant protection mechanisms are overwhelmed by excessive amounts of ROS. E.g. The process of neutralizing ROS in unsaturated oils use up the body's antioxidant supplies.
UNCONTROLLED (excess) highly chemically reactive ROS (or their metabolites) can damage the most oxidatively-sensitive cellular components, which can cause local injury, cellular malfunction, and eventual organ malfunction leading to a variety of diseases, or even death.
Polyunsaturated fatty acids (PUFAs) in cell membranes and lipoproteins (vehicles in which lipids travel in the bloodstream) are prominent targets of ROS attack . ROS damage to cell membrane structure can cause membrane dysfunction and altered receptor function; PUFAs in cell membranes and lipoproteins are key to bringing robust oxidation back to a low-energy producing or fermenting cell by providing electrons. However, PUFAs will readily give up an electron when attacked by ROS ( Hydroxyl Radical in particular),in a self-perpetuating process called lipid peroxidation (The breakdown of the resultant lipid hydroperoxides often involves transition metal ion catalysis)..
Numerous structurally and functionally disruptive effects include:
ROS damage proteins and nucleic acids (i.e. DNA, RNA). The oxidizing power of ROS degrades amino acids, deactivates enzymes, damages protein receptors, and readily attack nuclear and mitochondrial DNA if they are formed in its vicinity as seen in radiation, resulting in strand breaks and other types of DNA damage. Proteins and nucleic acids have less reaction sites than PUFAs for ROS reactions to propagate chain reactions, so damage occurs only if radicals accumulate (which is not likely in normal cells), or if the damage is focused on a particular site of the protein, such as if a protein binds a transition metal ion. Marx G, Chevion M; 1986 Stadtman ER, Oliver CN; 1991, or it must elude the repair systems before replication occurs leading to mutations in tissue and organs, which can lead to cancer. Cheesman and Slater, 1993
ROS attack red blood cells;
ROS-induced oxidative damages may be precursors to aging and diseases. E.g. cancer, heart disease, diabetes mellitus, atherosclerosis, rheumatoid arthritis, hypertension, sleep apnea, brain damage and neurodegenerative diseasese.
| Sources of ROS | ||
|---|---|---|
| Cellular Metabolism | Immune System Cells | Hyperglycemia |
| Irradiation | Excessive metals | Damaged fats |
| Smoke, smoking | Food Additives | Wearing synthetics |
| Caffeine | Cell phones | Non-fresh food |
| Radiation | Too much oxygen | Micro-waved food |
| TV screens | Deep-fried foods | High voltage cables |
| Smoke, smoking | Food Additives | Wearing synthetics |
| Bio-oxidative Therapies | Herbicides, pesticides | Environmental pollutants |
| Drugs, vaccinations | Psychological stress | Physical trauma |
ROS are a by-product of aerobic cellular energy production - ROS are produced in the body as a by-product when oxygen is used to produce energy from food components. As part of this process, ROSare formed as oxygen is chemically reduced along the electron transport chain (ETC) in the mitochondria (this accounts for 90% of cell's oxygen consumption).
ROS are leaked from the Electron Transport Chain (ETC). Both the mitochondria and endoplasmic reticulum leak a considerable number of superoxide radicalsgenerated from molecular oxygen, which under normal circumstances, is by far the main source of radicals that the body must deal with.
| Coenzyme Q generates superoxide (O2•-) |
|---|
|
- One of the major sites of O2 • - generation is the ETC which leaks ROS radicals in the form of semiquinone radicals of coenzyme Q. The 1-electron form of CoQ occasionally leaks into the inner mitochondrial membrane. The nonspecific interaction of a CoQH • with molecular oxygen results in the formation of a O2•- which abstracts an electron from some other molecule and initiates a free radical chain reaction. |
By electron transfer reactions in body (both enzymatically and non-enzymatically mediated
Red blood cells produce ROS during the binding and release of oxygen and carbon dioxide by hemoglobin.
The immune system white blood cells produce ROS when body reacts to an adverse factor. E.g. A wound, fever, nervous imbalance (stress), microbial infection or toxin. These conditions precipitate an inflammatory response, in which radicals, ROS, RNS or other reactive oxidants are released by immune system white blood cells (E.g. macrophages).
Emotional stress creates free radicals. Possibly today's main oxidation-causing stressor.
Infectious microbes such as bacteria, viruses, protozoa initiate an inflammatory process that leads to increased ROS production by phagocytes. E.g. infectious bacteria Chlamydia pneumoniae and the Herpes simplex virus have been proposed as initial inflammatory infectious agents in atherosclerosis.
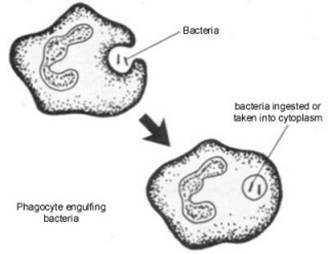
| PHAGOCYTOSIS in MORE DETAIL | ||||
|---|---|---|---|---|
|
During phagocytosis, phagocytic cell membrane enzymes (e.g flavoprotein cytochrome-b-245 NADPH oxidase enzyme system) are activated by exposure to:
to initiate a respiratory burst at the cell membrane, consuming large amounts of oxygen to produce Superoxide (O2 •- ). H2O2 is then formed from O2 • - by dismutation, with subsequent generation of OH• and HOCl by bacteria. Rosen H, Rikata R, Waltersdorph AM, Klebanoff S; 1987 H2O2 and O2• - are not reactive enough to destroy microbes themselves, and must be converted to OH•, 1O2*, HOCl or other oxidizing agent. However, the slow reactivity of H2O2 allows it to survive long enough to diffuse through microbial membranes and react with their lipids or DNA. Ozone (O3) is produced by antibody-catalyzed production of trioxidane from water and neutrophil-produced 1O2* |
Environmental toxins induce inflammatory response leading to damaging ROS and RNS. E.g. cigarette smoking by-products, exhaust fumes, household chemicals, heavy metals, pesticides/herbicides, certain drugs, radiation Pope et al. 2004; Suwa et al. 2002
| SOME MORE DETAILS ON TOBACCO SMOKE |
|---|
|
Hyperglycemia (chronically high blood sugar levels) induces oxidative stress in endothelial cells, which can cause an increase in the production of ROS (reactive oxidants, includes free radicals). Ceriello P et al, High Glucose Induces Antioxidant Enzymes in Human Endothelial Cells in Culture, Diabetes Vol 45 April 1996. PubMed
Hyperglycemia increases the formation of oxidized LDL. An important modulator in atherosclerosis and cardiovascular death.
Why the hyperglycemia?
Organs and tissues NOT dependent on INSULIN for their absorption of glucose are more susceptible to damage from periods of hyperglycemia than other organs. i.e. kidneys, blood vessels, peripheral nerves and lenses of the eye.
ROS are formed as necessary intermediates in a variety of enzymatic reactions
ROS are involved in intercellular and intracellular signaling. E.g. addition of superoxide orhydrogen peroxide to a variety of cultured cells leads to an increased rate of DNA replication and cell proliferation.
ROS production increased by EXERCISE / Some health problems. ROS production is higher during intensive physical exercise and with certain diseases such asdiabetes.
Oxidized Cholesterol
Cholesterol produced by the body or consumed in food is oxidized in the body. in its antioxidant role when it comes into contact with free radicals. (lipid peroxidation induced by ROS / RNS seems to be involved not only in cardiovascular disease, but also in cancer, rheumatoid arthritis, and other degenerative health problems, including accelerated aging).
Oxidized Polyunsaturated,Omega-6 and Omega-3 Fats. These "essential" fats are easily oxidized by ROS and RNS to become cell-damaging lipid peroxides. They are produced:
When we say oxygen "Oxidizes" food, what does that mean?
Ozone therapy, hydrogen peroxide therapy, chlorine dioxide therapy (CDT) and photodynamic Therapy (PDT). Deliberately introduce controlled amounts of ROS into the body.
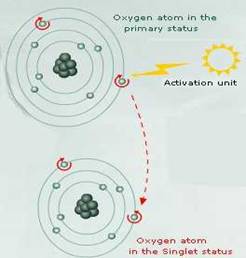
If atmospheric oxygen (3O2) absorbs sufficient energy to reverse the spin of one of its unpaired electrons it will form the ROS singlet oxygen in which its two unpaired electrons have opposite spins.
Singlet oxygen does not then have the spin restriction of triplet oxygen, and can participate in reactions involving the simultaneous transfer of two electrons (divalent reduction). Since paired electrons are common in organic molecules, singlet oxygen is much more reactive towards organic molecules than 3O2.
Singlet oxygen can be introduced into the body by various therapies E.g. Ozone therapy, Hydrogen peroxide therapy, Chlorine dioxide therapy (CDT), Photodynamic therapy (PDT)
In the body, ROS are more typically produced by the progressive reduction of Oxygen to form superoxide, hydrogen peroxide, hydroxyl radical and finally water.
The energy-producing cellular respiration chain in the mitochondria the cell energy "factories" ) uses > 90% of the body's oxygen, in a 4-step process that takes place one electron-transfer at a time, ultimately producing water, carbon dioxide (CO2) and energy. The summary process of how cells produce energy from glucose in the presence of sufficient oxygen is:
C6H12O6 (glucose) + 6 3O2 → 6 CO2 + 6 H2O + Energy
These partial equations show the intermediate ROS produced during this 4-step process:
|
1 |
3O2 + e- -→ O2 |
Superoxide Radical (mildly reactive) |
Adding 1e-to molecular oxygen |
|
2 |
O2•-- + H2O → HO2• + OH-- |
Hydroperoxyl Radical (highly reactive) |
Adding H to superoxide |
|
3 |
HO2• + e- + H → H2O2 |
Hydrogen Peroxide (poorly reactive) |
Adding 2 e- to molecular oxygen |
|
4 |
H2O2 + e- → OH• + OH-- |
Hydroxyl Radical (extremely reactive) |
Adding 1e- to Hydrogen peroxide |
A variety of enzyme systems are capable of generating significant amounts of radicals, including:
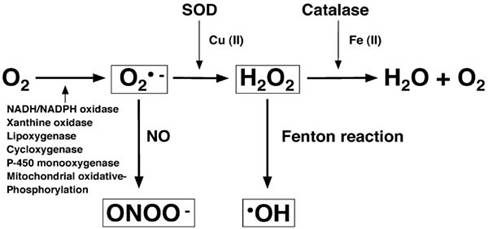
Autoxidation is a by-product of the aerobic internal milieu - molecules that undergo autoxidation include:
Autoxidation of any of the above molecules in a reaction results in the reduction of 3O2 and the formation of ROS -superoxide is the primary radical formed.
A ferrous ion (Fe II) also, can have its electron stolen from it by oxygen to produce superoxide and Fe III - by the process of autoxidation Fridovich, 1983 and 1995
Electrons are removed from glucose in a series of enzyme-assisted steps, which pass them along an "Electron Transport Chain"until they are finally accepted by oxygen, producing water. Glucose is the "reductant" in this redox reaction, since glucose "reduces"(gives away electrons to) oxygen.
Unsaturated lipids are particularly susceptible to oxidation, producing health-damaging / cancer-causing lipid peroxide ROS:
Chart of ROS and their metabolites in the body |
||||||
|
O2•-and H2O2 - are primary reactive metabolites of oxygen OH•, LOO•, LO• - are produced by secondary reactions of the primary metabolites. O2•- is far less damaging than H2O2, which penetrates membranes and can be transformed to OH•, HOCl, 1O2* |
||||||
|
Oxygen 3O2• DI-RADICAL |
How ROS is Formed from Oxygen |
Major Source (s) in Body |
Products Generated |
|||
|
Superoxide radical O2- Mildly Reactive Can not easily get into cells |
3O2 is reduced by addition of 1 electron to outer shells |
Electron leakage from mitochondrial Electron Transfer Chain: 3 O2 + e-→ O2- Phagocytic respiratory burst (uses NADPH-dependent Oxidase): NADPH +2O2 → NADP + O2-+ H2O2 Phagocytes also produce: OH• 1O2* NO* ONOO- |
Dismutation of O2•- O2•- + O2•- + 2H+ → H2O2 + 1O2 |
|||
|
Haber-Weiss Reaction - As reductant of free transition metals: E.g. iron-catalyzed (1) O2•- + Fe3+ → 3 O2• + Fe2+ (2) Fe2+ + H2O2 →Fe3+ + OH•+OH— |
||||||
|
H2O2 Poorly Reactive NON-radical
|
3 O2 is reduced by addition of 2 e's to outer shells |
Dismutation of O2•- O2•- + O2•- + 2H+ → H2O2 + 1O2*
|
Haber-Weiss Reaction (2) / Fenton Reaction - Main source ofOH• in presence of free transition metals: E.g. Iron-catalyzed (2) H2O2+Fe2+ → OH•+ OH—+Fe3+ |
|||
|
Production of HOCl by neutrophils: H2O2 +Cl-→ HOCl +OH— |
||||||
|
1O2* With hypochlorite or peroxynitrite H2O2 + ClGAS → 1O2*+ClGAS +H2O |
||||||
|
Hydroxyl RADICAL OH•
Most potent biological oxidant |
By reduction of H2O2 (i.e. by addition of 1 e-)
|
Haber-Weiss Reaction: O2•- and H2O2 in the presence of freetransition metals produce MOST OH•: E.g. Iron-catalyzed (1) O2•- + Fe3+ → 3 O2• + Fe2+ (2) Fe2+ + H2O2 → Fe3++ OH•+OH— Normally, most iron confined in RBCs
|
|
|||
|
O3and H2O2 |
2 O3 +H2O2→ OH• +3 O2• |
|||||
|
Radiation |
Radiobiological damage |
|||||
|
Singlet oxygen 1O2* Highly Reactive Excited NON-radical
Lowest excited state of oxygen
Passes easily into cells (because it is neutrally charged) |
TherapiesEnergy input (radiation) to 3 O2 Sensitizer with light and oxygen.(Basis of PDT for cancer) |
"Excited"electron spin flips + jumps to next orbital 3 O2 • +Energy → 1O2*
Chlorophyll (in plants), retinal, flavins, dyes (Bengal rose, methylene blue), natural pigment (porphyrins) Sens →Sens* → Sens + 1O2* uvO2 Photosynthesis may occur in skin cells |
Transfer the energy to a new molecule - thereby acting as a catalyst for free radical formation.
Reactions with substrate molecules - leading to the formation of a new free radical by oxidation. (Olefins, dienes, sulphides, aromatics, hetero-aromatics, terpenes, steroids, fatty acids, flavones, tetracyclines, vitamines, amino acids, proteins, nucleic acids, blood and bile pigments, and synthetic polymers)
(1) Ene reaction - H abstraction/O2 addition
Lipid peroxidation: RH+ 1O2* → ROOH Unsaturated lipid Hydroperoxide
(2) Cycloaddition - Typically results in: 1,2-dioxetaneorEndoperoxide
(3)Oxygenation - unfinished
1O2* reacts with ascorbate, producing H2O2 |
|||
|
Enzymatically catalyzed by peroxidases /lipooxigenasesor myeloperoxidase (MPO) - in neutrophils, monocytes, new macrophages. Or SOD |
H2O2w/hypochlorite or peroxynitrite H2O2 + ClGAS → 1O2* +ClGAS +H2O
HOCl with O2•- (MPO-dependent): HOCl + O2•- → Cl- + OH• + 1O2*
HOCl with H2O2: HOCl + H2O2 → HCl + H2O + 1O2* |
|||||
|
Dismutation of O2•- or ROO•: O2•- + O2•- + 2H+ → H2O2 + 1O2* (Non-catalyzed, rate pH-dependent)
O2•- + O2•- + 2H+ → H2O2 + 3O2 (Enzymatically w/SOD very fast reaction) ROO• + ROO• + 2H+ → RO• + 1O2* |
||||||
|
Endogenously |
Respiratory burst of phagocytes; By-product of metabolism |
|||||
|
Thermo- decomposition of dioxetanes |
Phosphite ozonides: (RO)3PO3 → (RO)3PO + 1O2* Endoperoxides: E.g. 9,10-diphenylanthracene peroxide (DAP)
|
|||||
|
Hydroperoxides (ROOH)/ Endoperoxides |
React with some metal ions. |
Involves breaking C=C bonds E.g. in carotene, chlorophyll, polyunsaturated fatty acids (PUFAs)
|
Produce Peroxyl ROO• /Alkoxyl Radicals RO• Vital for killer action |
|||
|
Ozone O3 |
|
O2•- as intermediate to OH•;
Peroxide anionO2 2-;
|
O3 oxidizes/ionizes organic molecules(saturated hydrocarbons, amines, sulfhydryl groups and aromatic compounds) to chiefly aldehydes, ketones, acids or alcohols.
O3 will oxidize metals (except gold, platinum, and iridium) to metal oxides (much easier to remove) - 2Cu2++2H++ O3→ 2 Cu3++ H2O+O2
O3 changes oxides into peroxides: SO2 + O3 → SO3 + O2•
O3 converts cyanides into cyanates (1000 X less toxic than cyanide): CN- + O3 →CNO- + O2• O3 → 1O2* + O1* Excited singlet oxygen MOLECULE 1O2* Excited oxygen ATOM O1*
|
|||
|
|
|
|
|
|||
| Relative Oxidation Power (Cl2 = 1.0) |
|---|
| (Cl2 = 1.0) |
|
Fluorine 2.23 Hydroxyl Radical (OH) 2.06 Atomic Oxygen (singlet) 1.78 Ozone 1.50 Hydrogen Peroxide 1.31 Perhydroxyl Radical (OOH) 1.25 Potassium Permanganate 1.24 Chlorine Dioxide 1.15 Bromine 0.80 |