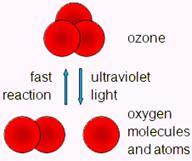
Ozone protects the Earth from harmful ultraviolet radiation. Oxygen in the stratosphere absorbs UV rays from the sun to become ozone, which is a blue gas - and actually the reason why the sky is blue.
Nature's wonderful self-cleaning system - Ozone's third oxygen atom (with a weak bond) detaches and begins a chain of reactions that produce powerful oxidants, which can combine with airborne pollutants and change them into less harmful compounds. Heavier than air, ozone falls earthward, oxidizing /destroying any pollutant it contacts. E.g. carbon monoxide and benzene.
Ozone is a Powerful Disinfectant and Toxin Remover -ozone has the ability to alter, decompose and/or neutralize materials that are toxic, without creating additional toxic compounds. Second only to fluoride as the world's most powerful oxidizer and sanitizer, ozone will eventually break down most chemicals into their natural components: H2O, carbon dioxide, sulfur, nitrogen and oxygen. Ozone has been used to clean water for large cities for over 100 years.
E.g. Formaldehyde (HCHO) + Ozone (O3) →CO2 (Carbon Dioxide) + H2O (Water) + O2
Ozone forms beneficial hydrogen peroxide (H2O2) in rain and snow - As a result of ozone in the upper atmosphere acting on water vapor, rain and snow both contain oxidant-producing H2O2. This is why plants grow better with rain water than with irrigation. Commercial horticultural growers actually add H2O2 to their irrigation water to produce healthier, disease free plants. H2O2 added to drinking water for farm animals has also demonstrated very positive results E.g.Cow's milk production is increased, with a lower bacteria count. Turkey and chicken growers report increased weight per bird using less feed.
Hydrogen Peroxide -Oxidative / Oxygenative Water
Many industries use ozone as an industrial oxidizer and sterilizer. Including industrial waste treatment, sewage treatment, aquarium sanitation, food preservation, sterilization of containers; The best known use of ozone is in water treatment because of its ability to kill bacteria and viruses. The EPA and the FDA acknowledge ozone's ability to oxidize 99.9992% of all waterborne pathogens.
| Examples of Ozone Applications | |
|---|---|
|
Chemicals |
Asphalt Fumes; Butane;Benzene; Gasoline; Toluene; Carbon Monoxide; Kerosene; Cigarette Smoke; Exhaust Fumes; Industrial Wastes; Creosote; Propane; Formaldehyde; Tetrachloride; Ammonia; Moth balls; Diesel fumes; Resins; Ether; Ethyl alcohol; Nicotine; |
|
Germs |
Viruses; Mold; Fungi; Bacteria; Algae; Mildew; |
|
Odors |
Cooking; Food; Garbage; Fecal, Paint, Onions, Garlic, Sewer Gases, Dead Animals; Bathroom; Decaying; Moth balls; Old Manuscripts; Furniture (incl. smoke damage); Coal smoke; Carpet; Fire; Animal; Body; Fish; Flood; Burned food; Smoking; |
|
Other |
Lactic acid; Rancid Oils; |