Ozone is an unstable, strong oxidizing gaseous molecule comprised of 3 oxygen atoms
In nature, ozone (O3 ) is formed by the action of UV radiation on oxygen (O2) - which causes the 2 atoms in an oxygen molecule (O2) to split up, after which each single oxygen atom joins another O2 molecule to make ozone (O3).
3 O2 + UV Radiation → 2 O3
Ozone can also be created when an electrical "spark" acts on oxygen. E.g. that "clean" smell you detect after a lightning storm is ozone.
In nature, ozone is created by Near UV radiation
3 3O2 → 2 O3 Exothermic reaction (light energy to heat)
Molecular Oxygen + 240nm< UV< 310nm → Ozone (O3) Absorbs UV radiation
Achieved in 2 Steps:
|
Step 1 - By PHOTOLYSIS: The OXYGEN MOLECULE is split into 2 EXCITED OXYGEN ATOMS O1* |
||||
|
3O2 |
→ |
O1* |
+ |
O1* |
|
|
240nm< UV< 310nm |
|
|
|
|
FREE DIRADICAL OXYGEN MOLECULE Electrons in lowest energy state |
Exothermic |
EXCITED OXYGEN ATOMS Technically written as O (1D) Each with 2 unpaired electrons and Absorbed "Splitting"energy make these HIGHLY REACTIVE w/excitation energy of 190 kJ/mol |
||
|
Step 2 - FORMATION of OZONE BY ELECTRONEGATIVITY: EXCITED OXYGEN ATOM joins an Oxygen molecule |
||||
|
O1* |
+ |
3O2 |
→ |
O3 |
|
|
|
|
|
See below |
|
EXCITED OXYGEN ATOM |
|
FREE DIRADICAL OXYGEN MOLECULE |
|
OZONE Formation of Ozone by Electronegativity |
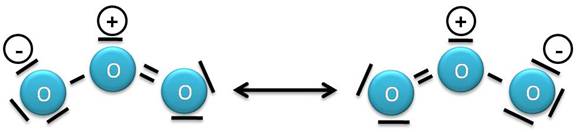
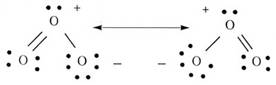
Ozone (Lewis Dot Diagrams)
Ozone is a strong oxidant. Oxidation occurs when any atom loses one or more electrons, giving it the ability to combine with other substances. E.g. an oxygen atom can combine with other substances to form water and gases.
- Oxidation potential of ozone is 2.7 - meaning it is much more reactive than oxygen, which has an oxidation potential of 1.23
Ozone strongly absorbs UV radiation to produce singlet oxygen MOLECULES and active oxygen ATOMS
O3 + hv (λ <= 313nm) → O2 (1 Δg ) + O(1D )
O3+ hv (λ <= 268nm) → O2 (1 Σ+ or 1 Δg ) + O(1D )
Ozone reacts with biological molecules to produce the reactive oxidant agent singlet oxygen 1O2* . Singlet oxygen is a name given to several higher-energy species of molecularO2 in which all the electron spins are paired.
Kanofsky JR, Sima P Singlet oxygen production from the reactions of ozone with biological molecules. J Biol Chem. 1991 May 15;266(14):9039-42. PubMed
Ozone reacts with the unsaturated fatty acids of the lipid layer in cellular membranes, forming hydro-peroxides. Lipid peroxidation products include alkoxyl RO▪and peroxyl radicals ROO▪, singlet oxygen 1O2*, ozonides, carbonides, carbonyls, alkanes and alkenes.
• If targeted cell membrane does not have sufficient protective antioxidant and antioxidant enzymes (SOD, CAT, GPx and reductase), it will be damaged and destroyed by reactive hydro-peroxide products - such as 1O2* , RO▪ and ROO▪. Healthy cell membranes contain protective antioxidant enzymes, many bacterial cell walls lack them.
E.g. Glutathione catalyzed by glutathione peroxidase reduces/neutralizes lipid hydroperoxides
Ozone is destroyed by hydroxyl radicals. The reaction converts ozone into oxygen.
O3 + OH• → O2 + HO2
HO2 + O3 → OH• + 2O2
EXCITED OXYGEN ATOM oxidizes water to hydrogen peroxide.
O1* + H2O→ H2O2
Ozone will oxidize most metals (except gold, platinum and iridium) to oxides of their metals in their highest oxidation state
E.g. 2 Cu+ + 2 H3O+ + O3 →2 Cu2+ + 3 H2O + O2
Ozone oxidizes nitric oxide to nitrogen dioxide:
NO + O3 → NO2 + O2
Ozone oxidizes ammonia to ammonium nitrate-but does not react with ammonium salts
2 NH3 + 4 O3 → NH4NO3 + 4 O2 + H2O
Ozone reacts with carbon to form carbon dioxide:
C + 2 O3 → CO2 + 2 O2
Ozone oxidizes sulfides to sulfates
PbS + 4 O3 → PbSO4 + 4 O2
Ozone gas reacts with hydrogen sulfide to form sulfur dioxide:
H2S + O3 → SO2 + H2O
Sulfuric acid can be produced from ozone, water and either elemental sulfur or sulfur dioxide:
S + H2O + O3 → H2SO4
3 SO2 + 3 H2O + O3 → 3 H2SO4
Ozone is unstable at high concentrations and decays to oxygen if it is does not contact something oxidizable - ozone has half-life of ~ 30 mins. in atmospheric conditions, but proceeds more rapidly with increasing temperature and pressure
2 O3 → 33O2
In 2steps:
O3 → 3O2 + O*
O*+ O*→ 3O2
Ozone depletion is caused by two non toxic, non flammable, normally very slowly or non reactive groups of gases: chlorofluorocarbons (CFCs) and halons. Used as refrigerant, propellants in aerosol spray cans, cleaning solvents in the manufacture of electronic circuit boards and as a blowing agent in the manufacture of foam insulation and furniture foam.
The CFC or halon molecule makes its way to the upper atmosphere where UV light can separate chlorine atoms from the CFC molecule and bromine from the halon. Once released, the chlorine or bromine molecule is capable of destroying ozone molecules in a continuous, repeating chemical reaction that allows a single chlorine or bromine atom to react with as many as 100,000 ozone molecules before finally settling out below the ozone layer
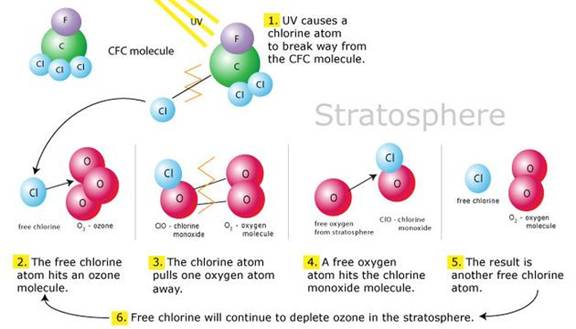
The process by which catalysts such as chlorine (E.g.
in the CFC molecule shown above), bromine, nitrogen oxide and hydrogen destroy atmospheric
ozone (O3).
Source
: Adapted from Hengeveld, 1991.