"The Body Electric"
(or Membrane Resting Potential)
Provides the "driving-force" for actively transporting ingredients required for cellular energy production across the cell membrane. This energy is then used to create a driving force across its mitochondrial membranes for "oxidative-phosphorylation"- the final, and most energy-yielding step, in the mitochondrion's energy production process
Affects All Electrical activity of the cell. The membrane resting potential preparesthe "excitable"nerve and muscle cells for the propagation of action potentials leading to nerve impulses and muscle contraction. E.g. the heart muscle cells (myocardial cells) require a sufficient membrane potential for the heart to beat; pain messages are passed via nerve impulses.
The membrane potential voltage controls the opening and closing of the potassium and sodium gates. Most ion channels are "gated"- stimulated to open or close by electrical (and sometimes mechanical or chemical) mechanisms. Stimulated by the membrane potential, the opening of the Na and K gates generates an inward current that affects the membrane potential itself (creating a reinforcing positive loop). Thus, the membrane potential controls the concentration and charge gradient of potassium and sodium ions either side of the membrane.
Unlike the "Resting" term implies, the cell is actually very busy keeping the unequal distribution of ions on each side of the cell membrane
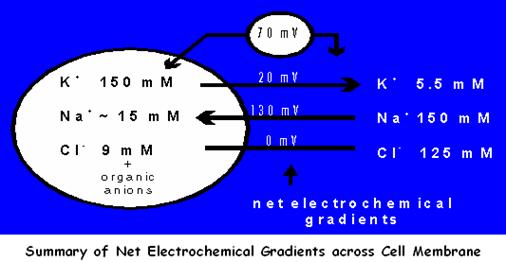
This is to establish a healthy cell membrane potential difference of about 70mV across the membrane, with the inside of the cell being more negative than the outside
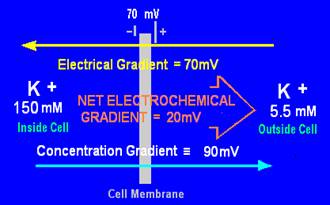
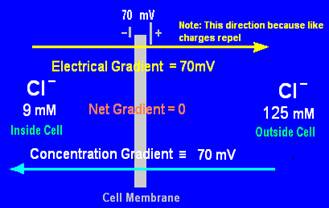
The concentration gradient of an ion is determined by the difference in concentrations (shown in diagrams in mM, which is mol/m3) of the ions in solution on each side of the membrane. Those concentrations have been converted here to their equivalent electrical gradient in millivolts (mV).
The largest net electrochemical gradient is 130mV, for Na+ ions into the cell
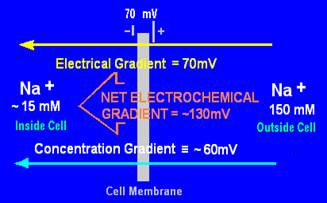
There is a small gradient of 20mV for K+ ions out of the cell
There is no gradient for Cl- ions
So therefore, this should mean that more Na+ ions diffuse into the cell than K+ ions diffuse out across the resting membrane.
But actually, this is not so, because we must also consider the membrane permeability, which is about 50 times more permeable to K+ than Na+. (i.e. Potassium can cross the membrane much more easily than Sodium).
| MEMBRANE PERMEABILITY |
|---|
|
The membrane's permeability to an ion refers to the ability of its membrane channels to conduct ions once they are open. This usually depends on the size of the ions in solution compared to the size of the channels.Most channels are large enough to pass the small hydrated K+ ion, but few will carry the larger hydrated Na+ ion across the membrane. |
The cell's resting "battery" voltage is established by the following 3 factors:
(1) The Preference of the Cell Membrane for Potassium to travel through it - More K+ ions diffuse out of the cell than Na+ ions diffuse into the cell. Since the membrane is much more permeable to K+ ions, there will be more K+ ions outside the membrane than can be compensated by the inflow of Na+ ions.
(2) Negatively Charged Molecules(Anions) are Trapped inside the Cell - Large negatively charged Molecules (here referred to as A- ) exist in the cytosol, which because of their molecular size, insolubility or bound position in the cytosol, cannot migrate across the membrane. Examples of these include proteins, DNA, RNAand ATP, all behaving as organic acids, giving off a hydrogen ion. When this positive ion is incorporated with oxygen to form water, it leaves the negative ion inside the cell.Cumulatively, these negative ions establish a negative charge trapped inside the cell.
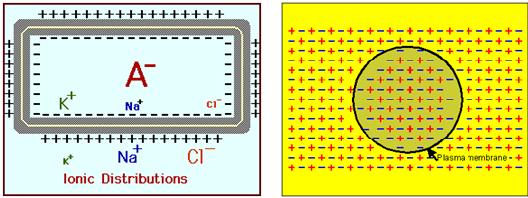
The negative anions trapped inside the cell, which cannot follow the K+ ions across the membrane, together with the excess of K+ ions diffused out of the cell over Na+ ions diffused into the cell, establish the resting potential.
The inside of the cell has a negative charge
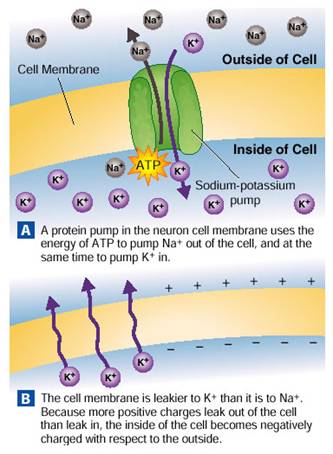
(3) Sodium / Potassium Pumps - Even though the cell membrane is not nearly as permeable to sodium as potassium, over time the slow diffusion of Na+ ions into the cell, together with the fast diffusion of K+ ions out of the cell will cause the cell membrane to lose its potential voltage by equalizing the charges on either side of the membrane.Eventually the cell membrane potential would drop to zero.i.e. the cell membrane "battery"would be dead!
Fortunately, the cell has a mechanism to bring back in the K+ ions and move out the Na+ ions - This mechanism is provided by the many energy-using Na/K pumps spanning the cell membrane, which continuously pumpNa+ ions back out of and K+ ions back into the cell, against their concentration gradients.The Na/K Pump uses ATP energy to simultaneously pump:
3 Na+ ions out of the cell and 2 K+ ions into the cell.
The Na/K Pump thus counters the potassium / sodium diffusion (leaking) out of the cell, and increases the membrane potential by pumping out of the cell one more sodium ion than it pumps potassium ions into the cell. The Na/K pump thus provides a mechanism by which Na+ ions are driven out of the cell faster than K+ ions are pulled in, keeping intracellular sodium levels low and potassium levels high.
This accomplishes several vital functions:
- It establishes an electrical potential difference (a "battery") across the cell membrane -with the interior of the cell being negatively charged relative to the exterior.
- Maintains osmotic balance. Sodium ions accumulated outside of the cell draw water out of the cell (otherwise, the cell would swell and burst from the inward diffusion of water).
- Provides energy for indirect pumps. Harnessing the gradient of sodium ions. E.g. to transport glucose into the cell.
Operating these continuously active Na/K pumps uses about 30% of the total ATP energy produced by the cell
The mitochondrial membrane uses positively charged hydrogen ions (Protons or H+) to create a strong membrane potential difference across the mitochondrial membrane - Hydrogen ions are maintained in a high concentration on the outside of the mitochondrial membrane by the action of the electron transport chain (See Cellular Respiration), which creates a mitochondrial membrane potential of about 40,000,000 volts/meter.
When this proton electricity flows back across the inner mitochondrial membrane it is used to power a molecular, enzyme motor called ATP syntase - which loads negatively charged phosphate anions onto ADP thus creating ATP.