• Too much Dietary / Supplementary Estrogen
• Overproduction of estrogen (ESTRADIOL or ESTRONE)
• Estrogen-Mimicking /stimulating xenoestrogens pervade modern day life
• Decreased PROGESTERONE production in the body
• Stress
• Obesity, Cardiovascular Disease, Digestive Problems, Insulin resistance
Any additional estrogen by prescription or supplemented, will increase estrogen levels and must be properly balanced with natural PROGESTERONE - Typical sources of prescription estrogen are birth control pills and hormone replacement therapy.
Over-consumption of phytoestrogens, especially soy or flaxseed adds to estrogenic load. Certain plants contain plant estrogens, which have an estrogenic effect when eaten to excess. Soybeans, soy products and flaxseed contain high levels of phytoestrogens. According to a report in The American Journal of Clinical Nutrition a small number of studies have shown that high levels of soy can increase menstrual cycle length, decrease FSH (follicle-stimulating hormone) and decrease LH (leutinizing hormone), affecting the reproductive cycle.
- High levels of soy generating anti-estrogeniceffect are equivalent to drinking three 12-ounce glasses of soy milk /dayfor a month - three 12oz glasses is 60g soy protein (equivalent to 45 mg of isoflavones);
- Excessive soybean consumption inhibits the thyroid and is widely associated with hypothyroidism - Asia has endemic hypothyroidism (China has 100,000,000 cretins).
Moderate phytoestrogen intake actually has an anti-Estrogenic effect - phytoestrogens "dock onto" estrogen receptors and have a weak estrogenic effect, preventing xenoestrogens and other estrogens from "docking" and having their stronger effect.
Women with diets containing moderate amounts of phytoestrogens excrete more estrogens into their urine and have lower blood estrogen levels.
- Liver must be able to eliminate unused excess estrogens - low/moderate intake of weak plant estrogens may replace our own more potent estrogens for receptor sites, but if the liver is incapable of eliminating them, then our own estrogens will still act if there are receptors to act upon.
It is now well-established that many phytoestrogens and hormone-disrupting chemicals exhibit an inverted-U "Dose to inhibitory response" curve |
|
Phytoestrogens and hormone-disrupting chemicals disrupt/inhibit hormones at low doses but not at high doses - What seems to happen is that the hormone system becomes overwhelmed and stops responding, so at high doses there is no observable effect. Traditional toxicological testing at high doses may miss important effects that only occur at lower doses. The Inverted U "Dose to inhibitory response" curve. Initially, as a dose rises, the inhibitory response rises. However, at some point as the dose continues to rise the response stops rising, then begins to diminish and falls back toward zero. 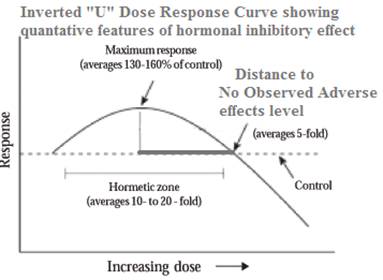 Examples of the Inverted U Dose-response curve: - xenoestrogenic BPA. A study of BPA found that low doses of BPA produced a greater inhibitory biological effect than higher doses. (EHP Vol. 109, Jul. 2001, pgs. 675-680.). - Phytoestrogens - EHP-published study demonstrates that: 1. Phytoestrogens at low doses inhibit the production of estrogen 2. At higher doses the inhibitory effect disappears and the phytoestrogens behave like estrogen itself, adding to the effect of the body's own natural estrogen - the authors say this may explain why low doses of phytoestrogens protect against breast cancer. [EHP Vol. 110, Aug. 2002, pgs. 743-748]. Estrogenic Pesticides. A study of adult male guppy fish, exposed to certain pesticides in their food (vinclozolin and DDE - both known to disrupt male sex hormones) exhibited shrunken testes, a significant reduction in numbers of sperm, and "a severe disruption in male courtship behavior." Some of the measured effects were greater at a lower dose, demonstrating an inverted-U dose-response curve [EHP Vol. 109, Oct. 2001, pgs. 1063-1070]. The authors of the guppy study did a literature search and found over 100 published papers reporting an inverted-U dose-response curve. |
TESTOSTERONE converts to ESTRADIOL via the aromatase enyme produced by fat cells;
Ovarian cysts or tumors. Can lead to excess estrogen production;
Obesity. All body fat has an enzyme which converts adrenal steroids to estrogen, so the more fat you have, the more estrogen is produced; this peripheral production of estrogen is the most common cause of excess estrogen
Murray RK et al. Harper's Biochemistry. 23rd ed. Norwalk CN:Appleton & Lange; 1993
Caffeine/Coffee/Alcohol/Nicotine. One study (Fertility and Sterility 2001;76:723-729) finds that drinking more than two cups of coffee daily may boost estrogen levels in women and could exacerbate conditions such as endometriosis and breast pain. The study included nearly 500 women aged 36 to 45 who were not pregnant, breast-feeding or takinghormones. All women answered questions about their diets, smoking habits, height and weight.Researchers measured the women's hormone levels during days 1 to 5 of their menstrual cycle.
Caffeine intake from all sources was linked with higher Estrogen levels. Regardless of age, body mass index (BMI), caloric intake, smoking, and alcohol and cholesterol intake.Women who consumed at least 500 mg of caffeine daily (E.g. 4 - 5 cups of coffee), had nearly 70% more estrogen during the early follicular phase than women consuming no more than 100 mg of caffeine daily (< 1 cup of coffee).
Smoking / Nicotine. Women aged 40 and older and those who smoked had higher levels of follicle stimulating hormone (FSH), which corresponds with fewer eggs remaining in a woman's ovaries. FSH tends to increase withage. Thus, the observation that smokers have higher FSH levels suggests that their ovaries are ``older'' than their chronological age.
Xenoestrogens (The Greek word xeno means foreign) are industrially made compounds with a molecular structure so similar to estrogen that they have estrogenic effects (i.e. stimulate estrogen receptors) in the body. xenoestrogens are having significant and serious effects on our health. Many endocrine-disrupting contaminants, even if less potent than the natural products, are present in living tissue at concentrations millions of times higher than the natural hormones. They are being blamed for increasing rates in hormone-sensitive breast, prostate and reproductive cancers, fibroids, cysts, reduced infertility, menopausal problems, PMS and early puberty in children.These endocrine disruptors create imbalances, especially in the reproductive, thyroid and adrenal systems. They can increase growth of the endometrium and disrupt the reproductive cycle. In the U.S., more than a quarter of all women are now having hysterectomies by the age of 60. Even men are not exempt from their effects, with many being diagnosed with BPH and complaining of declining sexual performance.
Xenoestrogens are strong and long-lasting. These chemicals bind permanently to receptor sites, accumulate in fat tissues, and disrupt nearly every biological process. Small daily doses build up, leading to an unbalanced endocrine system. Obesity and adrenal exhaustion (chronic fatigue/"stress") follow.
The environmental background of xenoestrogens provides a continuous estrogenic exposure. Unfortunately these modern day "Estrogen look-alikes"are nearly impossible to avoid since they are ubiquitously present in our lives, in meat and dairy, food preservatives, personal care products, household goods, herbicides, pesticides, fertilizers, plastics, and more.However, it is possible to reduce your exposure to xenoestrogens if you know where to find them, so brace yourself for a depressing revelation as these sources are revealed:
X-rated xenoestrogens - Endocrine Disruptors
PROGESTERONE is necessary to counterbalance estrogen and TESTOSTERONE
Women who do not ovulate during their cycle will not produce any PROGESTERONE that cycle. This is a common occurrence and worsens the already disturbed PROGESTERONE /estrogen balance;
PROGESTERONE production is reduced in menopause. Only the adrenal gland is producing it, because a woman is no longer ovulating.
| Symptoms of PROGESTERONE Deficiency | ||
|---|---|---|
|
• Recurrent early miscarriage |
• No period |
• Not ovulating |
|
• Endometriosis |
• PCOS |
• PMS |
|
• Cramps during menses |
• Blood Clotting |
• Swollen Breasts |
|
• Fibrocystic breasts |
||
Lack of available cholesterol for PROGESTERONE synthesis. The corpus luteum depends almost exclusively on peripheral low-density-lipoprotein-carried cholesterol (LDL-cholesterol)for making steroids. Gwynne, J.T.; Strauss, J.F., III: The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr. Rev. 3: 299 (1982).
Although such decreases in PROGESTERONE levels were not significant, it is likely that dosage of vitamin E greater than 600 IU will result in greater PROGESTERONE suppression. This may explain why PMT-A (most common type of premenstrual tension) symptoms worsened with 600 IU of vitamin E whereas 150 IU showed an improvement of PMT-A symptoms. London, R.S.; Sundaram, G.S.; Murphy, L.; et al.: The effect of alpha-tocopherol on premenstrual symptomatology. A double-blind study. J. Am. Coil. Nutr. 2: 115 (1983)
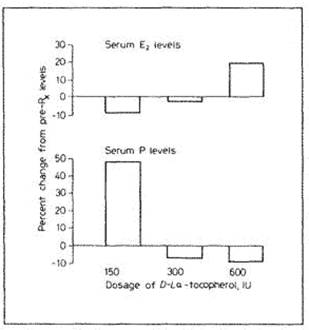
Inhibition of PROGESTERONE synthesis by the prostaglandin PGF2a. PGF2a is luteolytic (Degrades corpus luteum) in women
Dennefors BL et al. PROGESTERONE and adenosine 3', 5'- monophosphate formation by isolated human corpora lutea of different ages. Influence of human chronic gonadotropin and prostaglandins. J. clin. Endocr. Metab. 55: 102 (1982)
Cowan LD et al. Breast cancer incidence in women with a history of PROGESTERONE deficiency. Am. J. Epidem. 114: 209 (1981).
Wynder EL et al. Nutrition and the etiology and prevention of breast cancer; in Strax, Control of breast cancer through mass screening, pp. 89-100 (Littleton, Colorado 1979)
| Dr. Guy Abraham's thoughts on effects of Vitamin E on PGF2a | ||
|---|---|---|
|
The luteotropic effect (stimulating formation of corpus luteum of small doses of vitamin E (150 IU or less) could be due to the inhibitory effect of vitamin E on the release of AA from storage pool, in this manner decreasing the availability of PGF2a precursors. The net effect of vitamin E on PROGESTERONE synthesis would depend on the response of LDL-cholesterol and arachidonate release from storage pools to various dosages of vitamin E. It is possible that blockage of arachidonate release is more sensitive to vitamin E than suppression of LDL-cholesterol, such that low dose of vitamin E would decrease precursor availability for PGF2a synthesis (a luteotropic effect) and high dose of vitamin E would suppress LDL cholesterol (a luteolytic effect). |
||
Stress reduces PROGESTERONE function - when under stress, the adrenals pump out CORTISOL, which blocks some PROGESTERONE receptors and thus prevents PROGESTERONE function.
Chronic stress uses up our available PROGESTERONE. Chronic stress will cause adrenal fatigue, prompting the body to go to "Plan B"and use available PROGESTERONE to make CORTISOL. However, in this scenario, there is not enough PROGESTERONE to make the needed TESTOSTERONE for a woman's sexual response, never mind to oppose rising levels of estrogen. No wonder a woman feels lethargic and disinterested in sex when stressed!
It is not uncommon for women to experience menstrual cycles without ovulating as many as 10 years before menopause. In those cycles enough estrogen is made to create menstruation, but not enough PROGESTERONE to balance it. Even after menopause, the estrogen ESTRONE, ccontinues to be produced in the fat cells, while PROGESTERONE production is greatly diminished, since the ovaries are no longer producing and the job is left to the adrenal glands.
A healthier liver takes up, degrades and excretes excess estrogen, xenoestrogens and sex hormone-binding globulin (SHBG) in bile or urine. A weak liver that fails to eliminate these surpluses in the body is often responsible for hormonal abnormalities.
Excessive alcohol consumption. Blocks the liver enzyme that breaks down estrogen. Overindulging raises estrogen levels in both men and women;
Diseases of the liver. Cirrhosis (lesions in the liver) or decreased enzyme activity can lead to increased estrogen levels;

Vitamin B6, magnesium, zinc and/or B2 deficiency. B6 is an important cofactor for enzymes necessary for the liver to conjugate estrogensfrom the blood. A B6 deficiency affects the liver's estrogen clearance, keeping blood estrogen levels high.
Biskind, M.S.: Nutritional deficiency in the etiology of menorrhagia, cystic mastitis and premenstrual tension. Treatment with vitamin B complex. J. clin. Endocr. Metab. 3: 227-234 (1943).
Biskind, M.S.; Biskind, G.R.: Effect of vitamin B complex deficiency on inactivation of ESTRONE in the liver. Endocrinology 31: 109-114 (1942).
Biskind, M.S.; Biskind, G.R.: Inactivation of TESTOSTERONE propionate in the liver during vitamin B complex deficiency. Alteration of the estrogen-androgen equilibrium. Endocrinology 32: 97-102 (1945).
Biskind, MS.; Biskind, GR.: Biskind, L.H.: Nutritional deficiency in the etiology of menorrhagia, metrorrhagia, cystic mastitis, and premenstrual tension. Surgery Gynec. Obstet. 78: 49-57 (1944)
Abraham, G.E.; Schwartz, U.D.; Libran, M.M.: Effect of vitamin B-6 on plasma and red blood cell magnesium levels in premenopausal women. Ann. clin. Lab. Sci. 11: 333 (1981).
Magnesium also has a direct role in estrogen conjugation. By increasing the activity of an enzyme (glucuronyl transferase) involved in the liver's glucuronidation of estrogens
Abraham GE. Nutrition and the premenstrual tension syndromes, J Appl Nutr, 1984; 19:57-63

Aging and Certain Drugs. Impair liver function;
Iodine Deficiency causes estrogen imbalance. Iodine directly controls how much estrogen is produced by the body and in some cases the lack of iodine might cause a surge in the production of the estrogen;
Iodine supports natural cell death in the breasts and ovaries in a woman's monthly reproductive cycle - In preparation for pregnancy each month, there is a build up of cells in the breasts and uterus. When conception does not occur, the body rids itself of these then unneeded cells by a natural monthly "programmed cell death", called apoptosis.Not having appropriate monthly apoptosis can lead to fibrocystic breasts, polycystic ovarian syndrome or endometriosis.
Some studies supporting anti-Estrogenic effect of iodine
Stadel BV, Dietary iodina and risk of breast, endometrial, and ovarian cancer, Lancet,1976;
Typically seen in a diet heavy in artificial ingredients and refined sugar and flour
High fat, carbohydrate rich diet and resultant weight gain raises estrogen levels. An enzyme in fat cells converts adrenal hormones to Estrogens.